Coming back to cytologism: bone marrow stem cells
as the central factor in cancer.
Recent papers have highlighted the role played by stem cells in neoplasia, as exemplified by Houghton et al. (Science; Nov. 25, 2004)(1), who reported that solid cancers originate from bone marrow derived (bmd) cells instead of from resident cells of specific organs. These findings present a serious challenge to current working hypotheses on tumour initiation, progression and treatment. Conclusions reached from work using purposefully manipulated animal models, however, need to be regarded with caution. For example, in the mouse model studied by Houghton et al (1) most of the cells were derived from the bone marrow, but this may be peculiar to the ontogenesis of small mass, short-lived rodents. These tissues, which have short mitotic span phenotypes, have more in common with the embryonic type than the adult type of human tissues. In the former, there is a regeneration driven by bmd cells, while the last step, dealing with the formation of gastric cancer, consists of an interaction between a regenerative bmd field and bmd cells. In most human adult tissues, however, there is no regeneration; rather, there is repair and limited proliferation, achieved by non-bmd resident cells. In humans, support for the central participation of bmd cells in neoplasia comes from earlier theoretical models based on results from clinical histopathology(2) According to current opinion, most epithelial tumours originate from tissue-specific cells. The progressive accumulation of mutations in these resident cells, which takes place throughout life, triggers their transformation. In the axio-somatic model, malignancy is induced in these mitotic senescent cells by coupling with immigrant bmd cells. It is assumed that the nuclei of these bmd stem cells are essentially vectors of mobile, nonprotein-coding, selfish DNA(3), conveying mitotic potential to the mutant target. Pathologists who examine human tumours have not yet identified foreign stem cells within cancerous tissues. However, the presence in tumours of apoptosis-like phenomena, which have been attributed solely to resident tumour cells, may also be interpreted as being due to immigrant erythroblasts enucleating within carcinomas (photomicrograph). These occurrences have been found to coincide with transient periods of erythroblastemia in cancer patients. Early mathematical models predicted the existence of a central factor in cancer. The stepwise decreasing acceleration in cancer incidence observed after 50 years(4) may be linked to the age-related linear depletion of this forgotten axially based medullary stem cell pool that is presumably involved in malignancy. In analogy with the role played by fertilization in embryogenesis, the triggers are most likely in simple cell-to-cell interactions preceding malignancy. The spreading of bone marrow stem cells to preneoplasic fields, primary tumours and metastases in humans may be pivotal in initiating and sustaining tumour growth. If mathematical models in cancer need to be rebuilt, it seems urgent, according to the authors’ propositions,(1) to initiate new therapeutic approaches against oncotropic bmd stem cells in cancer patients.
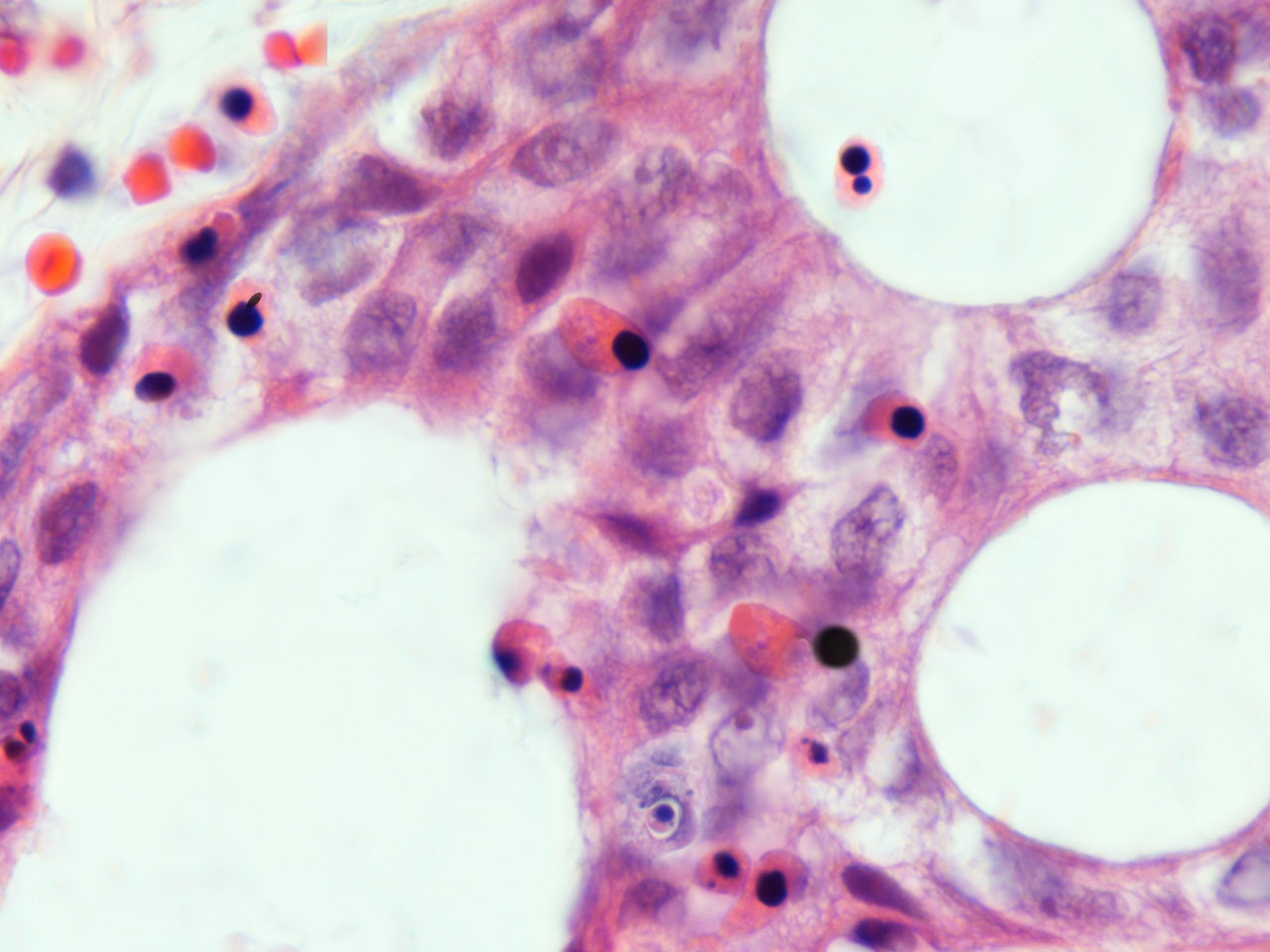
In human carcinomas, apoptoic-like phenomena played by resident neoplastic cells or by bone marrow derived cells immigrant to the field ? Left upper corner: Red cells and erythroblasts . Rest of the field: Enucleating erythroblasts.
Note the hematoxyphilia of heterochromatinic nuclei. Enucleating erythroblasts. Extruded naked nucleus and erythroid plastid.(acidophilic body). Apoptoic bodies. HE. 10 x 100. o.i.
Acid-fast nuclei in cancer.The same neoplasic field stained with an acid-fast method.Note the erythroblast nuclei and naked nucleus stained brilliant red with basic fuschin resistant to decoloration by acids and alcohol. Modified acid-fast method ; Berg´s method.
Santiago L Palacios-Llopis
sldpalacios@eresmas.net
Profesor Asociado. Dpt. Morfologia. Universidad Las Palmas GC.
Anatomia Patológica. Hospital General de Lanzarote. Carretera de Tinajo s/n. Lanzarote. 35500 Las Palmas. Spain.
1 Houghton JM,Stoicov C, Nomura S et al. Gastric cancer originating from bone marrow derived cells. Science 2004; 306: 1568-71.
2 Palacios SL . The axio-somatic model in tumor and embryonic development. Med Hypotheses 1994; 43: 86-92.
3 Dawkins R 1976. The selfish gene. Oxford: Oxford University Press.
4 Frank SA. Commentary: Mathematical models of cancer progression and epidemiology in the age of high throughput genomics. InternatlJEpidemiol 2004; 33:1-3.
5 Palacios-Llopis SL. Evidence for the erythroblastic origin of hematogones in humans. Pathology International. 2004; 54s2: A382


